Not many of Leucippus's books are around today, but he did write some, like "The Great World System." And here's the cool part: scientists today say Leucippus was right! Everything is made of atoms, just like he said.
Leucippus and Democritus's Vision of Atoms
Over two millennia ago, philosophers Leucippus and Democritus proposed ideas that still shape science today:
1.They believed everything comprises tiny, indivisible "atoms."
2. Atoms move within an open space, termed the "void."
3. When atoms collide, they might bond or deflect.
4. Distinct materials possess unique atoms varying in shape and size.
5. An atom's structure dictates an object's properties, like its taste or texture.
These insights, while ancient, remain foundational in our modern study of matter. The depicted portraits serve as a tribute to these visionary thinkers, reminding us of the profound impact their theories continue to have. As we proceed, we'll explore how these early concepts compare with today's atomic understanding.
The Atomic Ideas of Democritus
Democritus, was a prominent Greek philosopher active between 460 BC to 370 BC, and renowned for pioneering the concept of atomic theory. Under the guidance of his mentor Leucippus, he envisioned a universe where "atomos," Greek for indivisible, existed. These eternal atoms, specific to their material, could not undergo destruction. For example, he proposed that a stone's atom remains unique and distinct from all other substances. Remarkably, this ancient term "atomos" evolved into the English word "atom."
A masterpiece painted in 1746 by Charles Antoine, "The Cheerful Democritus," vividly captures the philosopher's zest for life. While centuries have passed, Democritus's groundbreaking insights into the atomic world still hold the spotlight in modern scientific discourse. Considering he sculpted these theories over two millennia ago, the lasting impact he's made on our understanding of the universe truly instills a sense of wonder
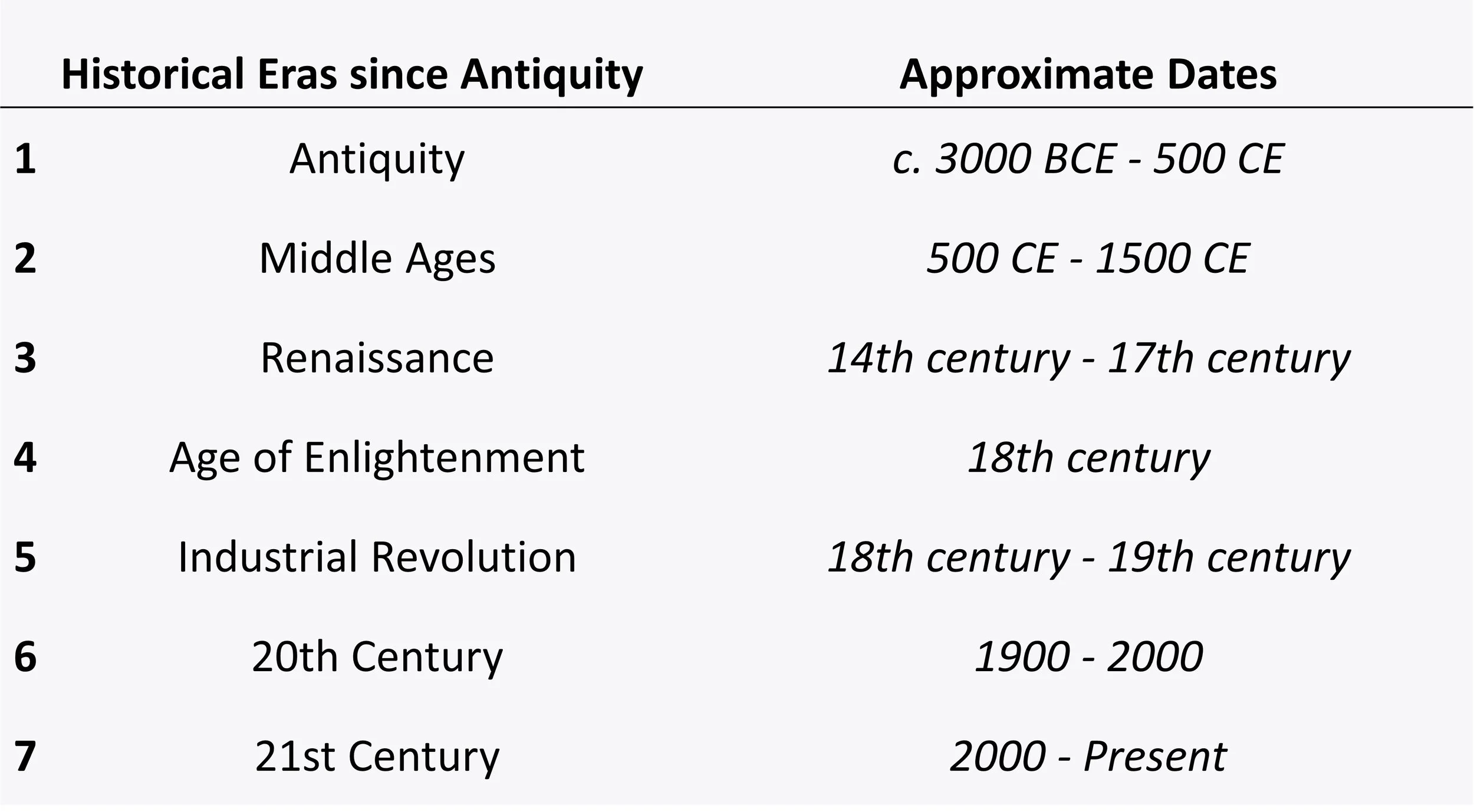
Our Journey Through Time & Atoms
This slide shows a table breaking down seven essential periods or "eras." Like chapters in a book, each era tells a part of the story about how people learned about atoms. Starting with ancient thinkers in the "School of Athens" and moving to today, we'll see how our understanding of atoms has changed and grown. As we go through each era, you'll notice that discoveries about atoms come faster and become more exciting. This table will guide us as we explore the fantastic world of atoms through history. Get ready to journey through time and learn how tiny atoms have a big story to tell!
Discovering Atoms: Inspired by Art and History
Have you ever seen Raphael's painting, "The School of Athens"? It's a big picture showing ancient Greek scholars talking and thinking. In the center, Plato and Aristotle are discussing big ideas. Some of these thinkers, like Leucippus and Democritus, believed everything was made up of tiny, unbreakable parts called "atomos." This idea started our journey to learn about atoms. Raphael's painting gives us a look into the past when these big ideas were born. Our course will take you from these early talks all the way to the cool science labs of today. With Raphael's painting as our starting point, let's dive in and uncover the amazing story of atoms, step by step!
The blog is written for a middle school audience, ages 11-13.